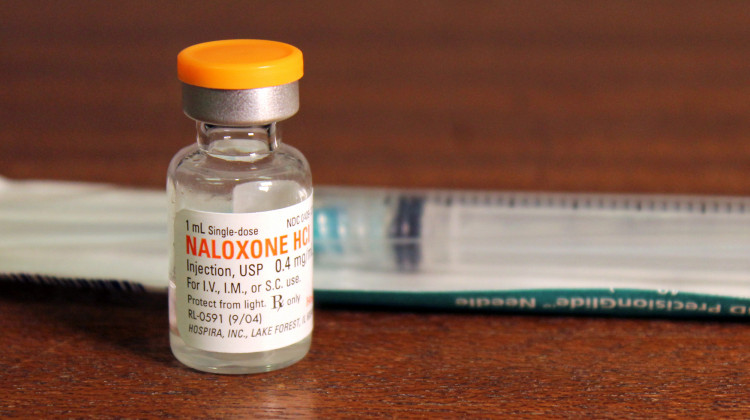
Hospira, one of the manufacturers of the opioid overdose antidote naloxone, issued a voluntary recall of its single-use cartridge syringe system.
Lauren Chapman/IPB NewsNaloxone manufacturer Hospira issued a voluntary recall of its single-use cartridge syringe system for the opioid overdose antidote.
The company says it found loose or embedded particulate matter on the syringe plunger.
The recall is on lot numbers 72680LL and 76510LL.
If someone is exposed to the particulate, Hospira says there is a low chance of experiencing adverse health effects, including allergic reactions and pulmonary dysfunction. The company says it has not received any complaints about the items affected by the recall.
A representative from the Indiana State Department of Health said none of the naloxone distributed by the department is part of the recall.
This story has been updated.
 DONATE
DONATE







 Support WFYI. We can't do it without you.
Support WFYI. We can't do it without you.